Abstract
Targeted eradication of transformed or otherwise dysregulated cells using monoclonal antibodies (mAb), T cell engagers (TCE) or chimeric antigen receptor (CAR) cells is very effective for hematologic diseases. CD123, the interleukin-3 (IL-3) receptor alpha-chain, is highly expressed in various hematological malignancies, including acute myeloid leukemia (AML) and blastic plasmacytoid dendritic cell neoplasm (BPDCN). However, shared expression of CD123 on hematopoietic stem and progenitor cells (HSPCs) bears the risk for extensive myelotoxicity upon targeted depletion. To enable therapeutic targeting of non-essential proteins post HSC transplantation (HSCT), it was proposed to engineer HSPCs that lack the target protein. Here, we show that expression and function of a genetically modified CD123 receptor can be preserved in genome engineered therapeutic cells despite full shielding from targeted therapy.
We used the known co-crystal structure of CD123 bound to the mAb CSL362 (talacotuzumab) for computer-aided protein engineering. In silico, 28 candidate single amino acid (aa) substitutions at 3 key residues were designed to interfere with CSL362 binding while potentially preserving its function. Flow cytometry confirmed no binding of a CSL362 biosimilar to 13 variants and weak binding to 12 variants while 3 variants showed similar binding as wildtype (wt) CD123. Protein expression of all variants was comparable to wt CD123. Using real-time, label-free protein interaction measurements, we could not detect interactions between the non-binding variant receptors and the CSL362 biosimilar, even at high protein concentrations. Despite this, the non-binding variants preserved the normal binding of IL-3. Protein characterization of selected non- and weak binding variants demonstrated normal biophysical properties of all but one variant.
All 13 non-binding variants abolished antibody dependent cellular cytotoxicity (ADCC) of the CSL362 IgG1 biosimilar in a surrogate reporter assay. In addition, a CSL362-derived TCE resulted in T cell activation and target cell killing of wt and strong binding CD123 variants while non-binding variants neither activated the T cells nor resulted in cell killing. Finally, the majority of non-binding variants (12/13) were completely protected from CSL362-CAR T cell cytotoxicity.
To assess the function of the variant receptors we engineered the top 2 variants into the IL-3 dependent cell line TF-1 using CRISPR/Cas9-mediated homology directed repair (HDR). Engineered variant TF-1 cells demonstrated IL-3 dose-dependent growth comparable to their unedited counterparts. The addition of the CSL362 biosimilar inhibited IL-3 dependent cell growth in unedited TF-1 cells but not cells expressing variant receptors.
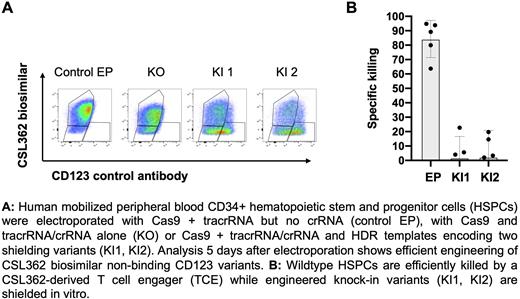
Similarly, CRISPR/Cas9-engineered human CD34+ HSPC knock-in (KI) cells expressing the selected variants (Fig. 1a) were shielded from TCE killing using HLA-matched T cells in vitro (Fig. 1b). In addition, a set of in vitro experiments performed with CD34+ HSPC KI cells suggested normal differentiation potential and intact IL-3 signaling as measured by pSTAT5.
In summary, our data demonstrate that single aa substitutions in CD123 introduced to HSPCs by CRISPR/Cas9-mediated HDR enable selective shielding from various immunotherapies, while preserving biophysical and functional properties. Variant IL-3 receptor engineered HSPC's and paired immunotherapies could enable novel and targeted therapeutic interventions post HSCT to either treat minimal residual disease or be used as a salvage therapy. Such approaches could expand the therapeutic window and broaden indications and applications for targeted immunotherapies for various malignant and benign conditions.
Disclosures
Wellinger:Roche: Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.